Conservation of Energy
The principle of the conservation of energy states that energy can neither be created nor destroyed. If a system undergoes a process by heat and work transfer, then the net heat supplied, Q, plus the net work input, W, is equal to the change of intrinsic energy of the working fluid, i.e.
t initial and final states, respectively. The special case of the equation applied to a steady-flow system is known as steady-flow energy equation. Applying this general principle to a thermodynamic cycle, when the system undergoes a complete cycle, i.e. U1 = U2, results in:
Q= The algebraic sum of the heat supplied to (+) or rejected from (-) the system.
W= The algebraic sum of the work done by surround
ings on the system (+) or by the system on surroundings (-).
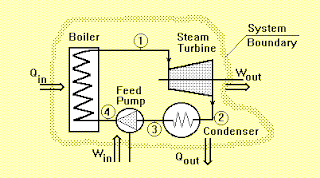
Applying the rule to the power plant shown in figure below,
gives:
Q = Qin - Qout
W = Win - Wout
Qin + Win - Qout - Wout = 0
where,
Qin = Heat supplied to the system through boiler,
Win = Feed-pump work,
Qout = Heat rejected from the system by condenser,
Wout = Turbine work.
In its simplest form, the First Law of Thermodynamics states that neither matter nor energy can be created or destroyed. The amount of energy in the universe is constant – energy can be changed, moved, controlled, stored, or dissipated. However, this energy cannot be created from nothing or reduced to nothing. Every natural process transforms energy and moves energy, but cannot create or eliminate it.This principle forms a foundation for many of the physical sciences.
The First Law of Thermodynamics is one of the absolute physical laws of the universe. Everything in the entire universe is affected by this law, as much as time or gravity. There are three Laws of Thermodynamics. The Second Law (Increased Entropy) and the Third Law (Zero Entropy at Zero Kelvin) are dependent on the First Law and each other. Together, these laws form part of the baseline for all modern science. No exceptions or contradictions to these laws have ever been observed.
Energy that enters a system must either be stored there or leave. A system cannot output more energy than it contains without an external source of more energy. This energy can be in work, heat, potential, or kinetic form. On a small scale, this can be explained this way: “Change in internal energy equals the difference of heat transfer into the system and the work done by the system.” On a large scale, this Law is still observable. Oceans and planets and solar systems all operate under the control of the First Law of Thermodynamics.
Some aspects of the First Law of Thermodynamics can seem confusing. A burning log in the fireplace seems to violate the principles of conservation of matter/energy. Burning the log appears to create energy and destroy matter. In reality, the energy and matter are only changing place and form, they are not being created or destroyed. The wood in the log has chemical potential energy, which is released when it is burned. This released energy appears in the form of heat and light. The matter of the log is changed into smoke particles, ash, and soot. The log’s total energy and mass before burning are the same as the mass and energy of the soot, ash, smoke, heat and light afterwards.
Rubbing your hands together generates heat on your palms. This is not actually creating energy. The work of your muscles takes the chemical energy of your body, changes it to work energy in your muscles, and then into friction (heat) energy in your skin. You’ve actually moved energy from your body through your muscles and into your hands. The First Law of Thermodynamics requires that the total energy of your body, muscles, and palms is the same both before and after you rub them together.
One example of how the First Law of Thermodynamics acts is the perpetual motion machine. No one has ever built a machine that can continue to move forever without any external energy source keeping it moving. Every machine requires some input to continue moving. This input can be wind, chemical reactions, magnets, and so forth. The reason that machines cannot move indefinitely is friction. No matter how good the lubrication or bearings are, every machine has to lose some energy to friction as it moves. This energy must come from the total energy of the system, as the First Law of Thermodynamics demands. This is why a bicycle wheel will eventually stop spinning unless you push it. This is why a car cannot coast forever on a flat surface. Friction demands some energy from the system. For a machine to run forever without any external source of energy, it would have to violate the First Law of Thermodynamics by creating energy out of nowhere.
Science has shown that the First Law of Thermodynamics applies to all matter and energy, no matter how much or what the conditions are. Looking at bigger and bigger systems of matter and energy eventually leads to a question: where did all of the matter and energy in the universe come from? The Second Law of Thermodynamics shows us that all of the energy in the universe is moving towards a less “useable” form. However, the First Law of Thermodynamics shows us that nothing in the physical universe can create or destroy that matter or energy. If nothing natural can create matter or energy, then something supernatural must have created them.